Requirements of 21 CFR Part 11
The FDA mandates that businesses maintain their records electronically. Companies utilizing a closed system for recordkeeping must comply with the requirements outlined in 21 CFR Part 11.10. Such businesses are responsible for implementing procedures and controls that protect the authenticity, integrity, and confidentiality of electronic records.
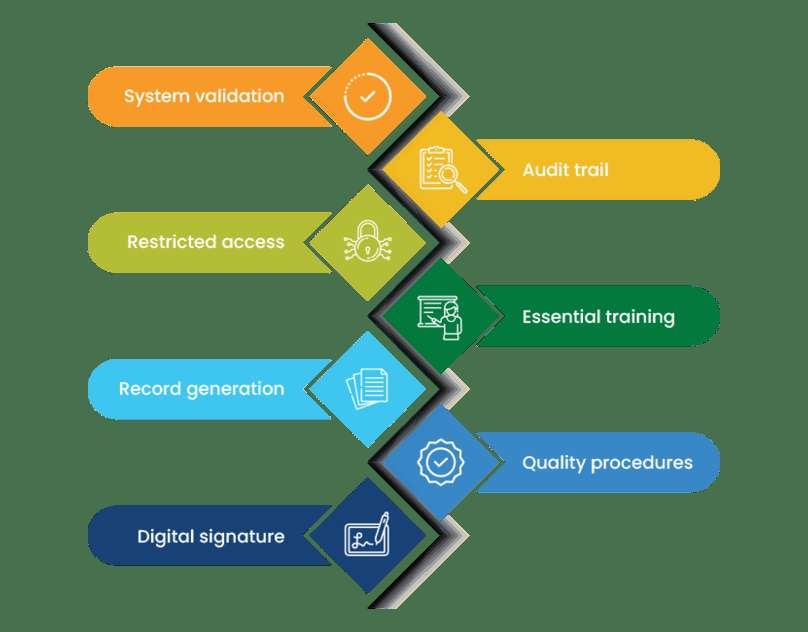
Key requirements include:
1. Validating the system to ensure the security and integrity of stored data
2. Maintaining an audit trail to document the evolution of processes
3. Restricting system access to enhance security and control
4. Ensuring all users receive proper training to effectively perform daily tasks
5. Enabling record creation with robust search and indexing capabilities for quick retrieval
6. Establishing quality procedures to maintain operational control over personnel and processes throughout the development cycle
7. Utilizing digital signatures across the organization to streamline and expedite the approval process
For more Info, Please Visit: https://www.compliancequest.co....m/21-cfr-part-11-com